Are you a manufacturer, authorised representative or importer of a medical device? If so, you will soon (most likely in May 2022) need to register information about yourself, your company and the medical device in EUDAMED. EUDAMED is the ‘European Database on Medical Devices’. In this article, you can read more about this database.
On 26 May 2021, the Medical Devices Regulation (MDR 2017/745) entered into force. A key objective of this regulation is to increase patient safety in the European Union. Therefore, for instance, one central database is being introduced in which all information on medical devices is collected: EUDAMED. EUDAMED is an existing system, which is currently being updated and adapted.
An important objective of EUDAMED is to improve the traceability of medical devices. It should also facilitate information exchange between market players, authorities and Member States and provide the public and healthcare professionals with better information on devices. Manufacturers, authorised representatives and importers of medical devices will soon be obliged to register in this database and to provide information about their medical devices. These obligations, however, do not apply to distributors.
Are you a manufacturer, authorised representative or importer of a medical device? If so, in order to gain access to EUDAMED, you must first apply for a Single Registration Number (SRN) in the EUDAMED actor module. You can do this right now.
Your application will be checked in the EU Member State where you are established. In the Netherlands, the Central Information Unit on Healthcare Professions (Centraal Informatiepunt Beroepen Gezondheidszorg) (CIBG) is the competent authority for this. The CIBG will approve your application once the entered data are complete and correct, and EUDAMED will subsequently grant you a Single Registration Number (SRN).
As indicated, you can only access EUDAMED with an SRN. As soon as EUDAMED is put into use, you need this access in order to:
Besides manufacturers, authorised representatives, importers and notified bodies, regulatory bodies will also have access to EUDAMED. In the Netherlands, these are the Ministry of Health, Welfare and Sport (VWS) and the Healthcare and Youth Inspectorate (IGJ). In addition, healthcare institutions, healthcare providers and citizens can also access the publicly accessible components of EUDAMED. They are free to look up information about medical devices, side effects and incidents, but are unable to register anything themselves.
Originally, EUDAMED was to be put into service simultaneously with the entry into force of the MDR on 26 May 2021. However, at the moment, EUDAMED is still under development. Nevertheless, a number of modules are already ready, and you are free to use these on a voluntary basis. These are modules for actors, UDI/apparatus registration and notified bodies and certificates.
In anticipation of further implementation, the European Commission adopted implementing regulation 2021/2078 in November 2021. In this regulation, which entered into force in December 2021, the Commission laid down the rules necessary for the establishment, access, maintenance and security of EUDAMED.
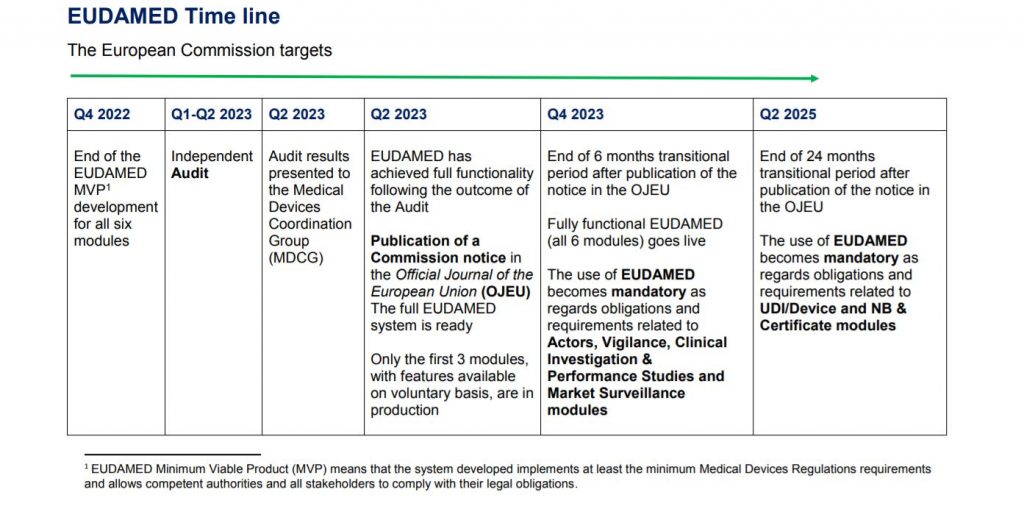
The remaining modules (clinical research and performance studies, vigilance and market surveillance) are currently still under development, and will be released once EUDAMED is declared fully functional. An independent audit will first be conducted for this purpose. It is expected that this audit will take place in the first quarter of 2023, after which the results will be published in the second quarter of 2023. The use of the various modules of EUDAMED will then become mandatory on dates to be announced in the Official Journal. The European Commission is currently aiming for dates between the fourth quarter of 2023 and the second quarter of 2025. Certification Company will keep you informed of the exact dates, of course.
5/10/2024
5/10/2024

5/10/2024
5/10/2024
5/10/2024
5/10/2024
5/10/2024
5/10/2024
5/10/2024
5/10/2024